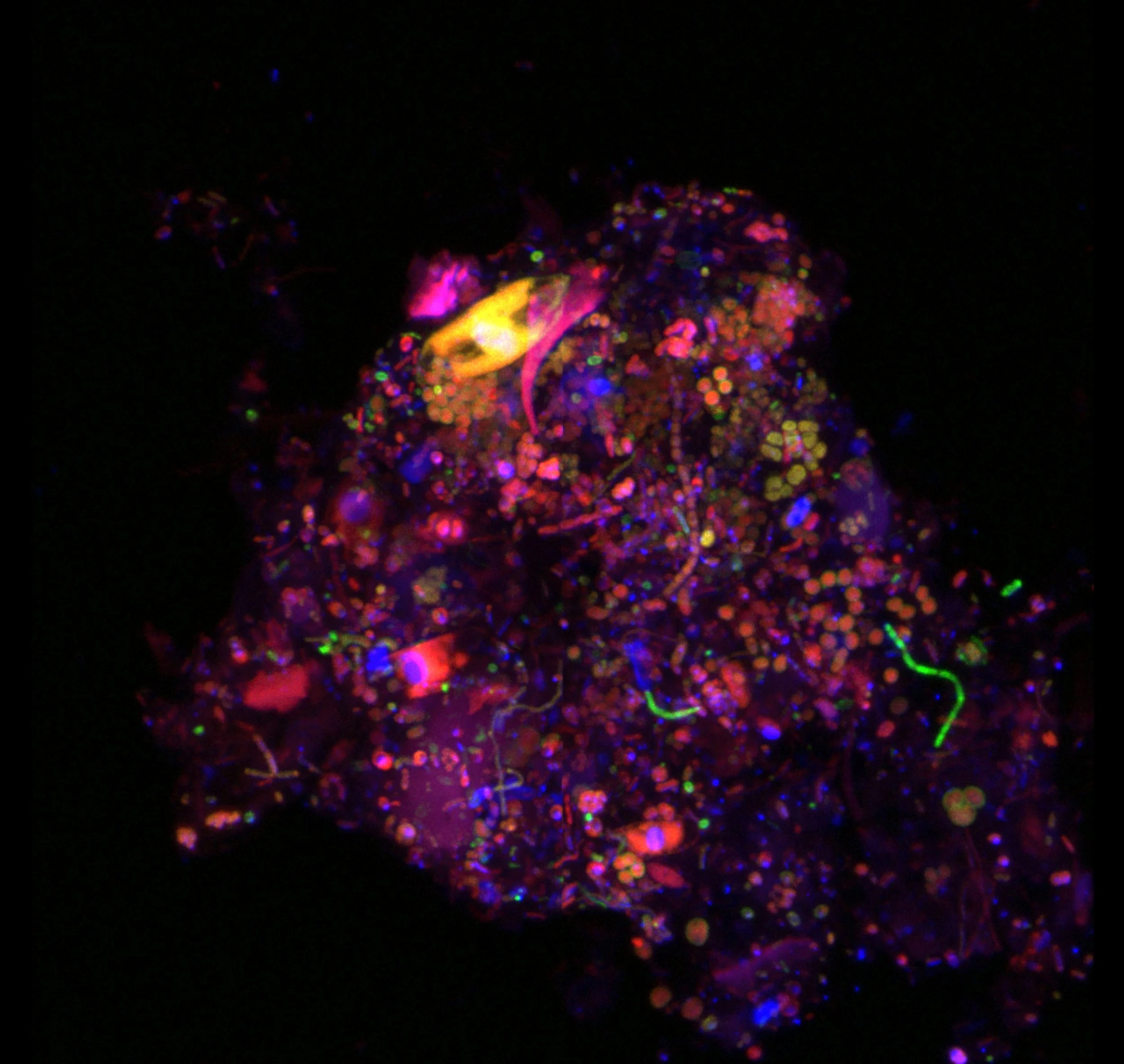
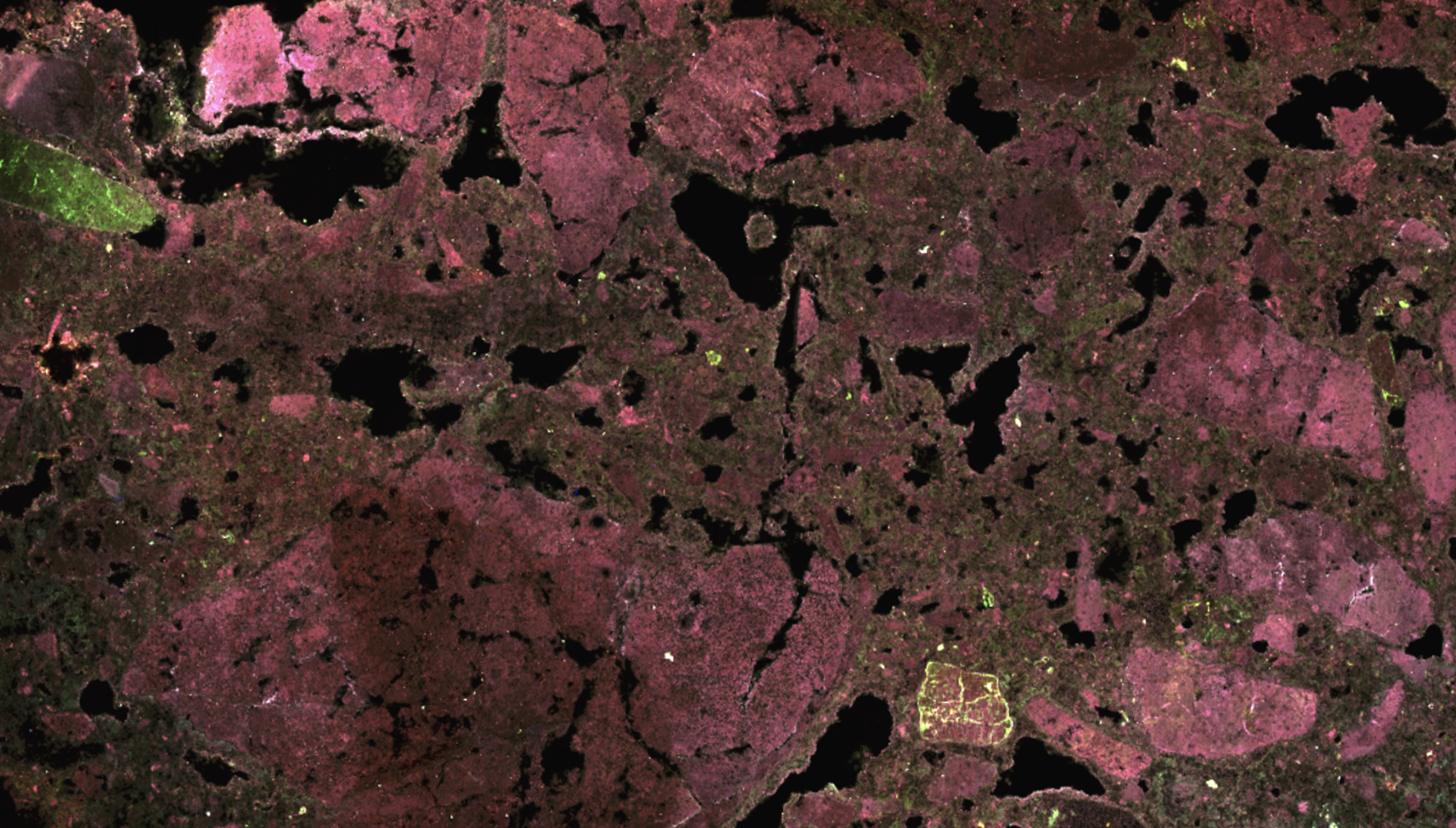
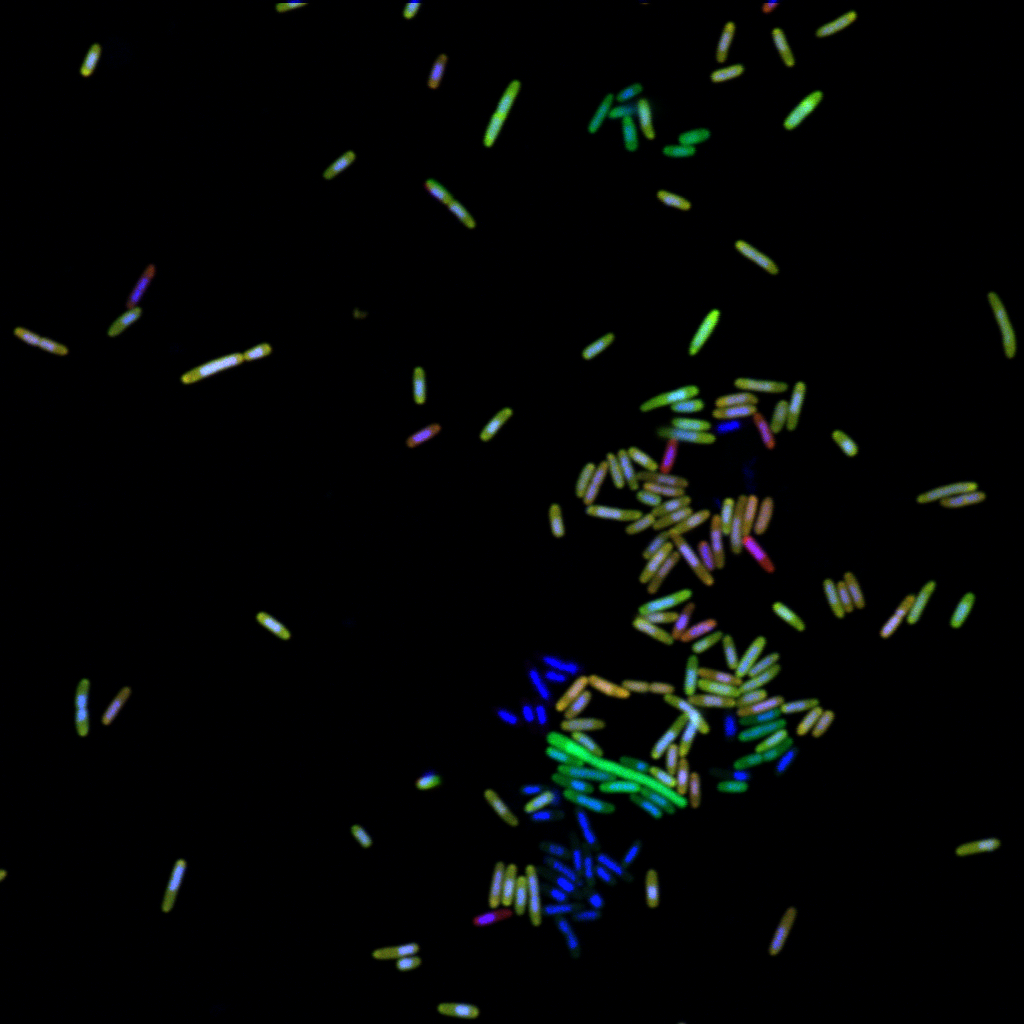
Our research uses advanced microscopy and molecular methods to understand the links between microbes and their mineral habitats
Revealing & characterizing new methane sinks in the Deep Sea
At marine methane seeps, an estimated one-fifth of the planet’s methane streams out of the Earth’s crust and into seafloor sediments, representing a critical control point for an important greenhouse gas. As a metabolic by-product of the anaerobic oxidation of methane (AOM), authigenic carbonate rock precipitates, frequently leading to extensive pavements and mounds. This potentially voluminous habitat had not been previously examined as an active microbial habitat, but after an initial characterization at Hydrate Ridge, Oregon, we found the phenomenon to be pervasive at all seeps sampled to date. Current work in the lab is characterizing the microbial community and methane-oxidizing potential of sediments and carbonates from newly-discovered methane seeps off the coast of Chile.
Microbial Colonization on Minerals
Understanding where microbes are found in a certain environment and what they are doing is crucial to revealing their functions and roles in biogeochemical cycles. However, it remains unknown if microbes preferentially colonize areas with higher mineralogical complexity and if so, whether they follow certain patterns, such as associating with metal-based minerals, which may serve as electron donors or acceptors. To test the hypothesis that microbes ‘choose’ areas of higher mineralogical complexity/heterogeneity, we use confocal microscopy, Raman spectroscopy and X-Ray fluorescence spectrometry to study the mineralogical composition and biomass localization in rock-hosted microbial communities coming from two extreme environments, The Cedars in California and the Basque Lakes in British Colombia. Our results provide a general framework for understanding microbial biogeography on the microscale, informing our understanding of biogeochemical cycling and the search for life beyond Earth.
Geomicrobiology of Polymetallic Nodules
Polymetallic nodules on the abyssal seafloor are abundant, ancient substrates with high abundances of industrially relevant minerals such as cobalt, iron, and nickel. They also host prolific microbial communities, though their role in mediating nodule formation - as well as other ecologically important elemental and nutrient cycles - is poorly understood. As a part of the collaborative Dark Oxygen Research Initiative, supported by the Nippon Foundation, we will conduct metagenomic surveys, as well as in situ and lab-based stable isotope probing experiments on nodules and sediments from the Clarion-Clipperton Zone, to disentangle the role of specific microbial taxa in mediating biogeochemical cycles. With a more complete understanding of how microbes and minerals interact to form nodules and shape elemental dynamics in the abyssal ocean, researchers, companies, and policymakers will be better positioned to make sustainable decisions about our relationship with the deep sea.
Dual-BONCAT: Multiplexing Substrate Analog Probing
Microbial communities are complex and dynamic, with different groups of microbes active under distinct conditions. Bio-Orthogonal Non-Canonical Amino Acid Tagging (BONCAT) uses synthetic amino acids to tag newly made proteins, allowing researchers to see and identify the active subset of a community. While BONCAT studies to date have used a single synthetic amino acid to evaluate cell activity in a single experimental context, we’ve recently developed a new approach, “dual-BONCAT,” using two synthetic amino acids to track differential responses to changing conditions. After validating the approach with E. coli, we deployed it in a salt marsh sediment community, finding that organisms potentially feeding on plant root sugars were more active during the day, and microbes likely metabolizing sulfur were more active at night. Dual-BONCAT offers an important advancement in multiplexing substrate-analog probing techniques, providing a more realistic understanding of metabolic activity under distinct environmental conditions.
Microscale Alteration Features in Ancient Stromatolites
The origin and early development of life on Earth are difficult to study. Dynamic geological processes continually recycle Earth’s lithosphere, restricting our interpretations of this critical interval. Stromatolite reefs—sedimentary structures mediated by microbial communities—represent some of the earliest fossil evidence for life. Specifically, the 2.7 billion year old Tumbiana stromatolites from Western Australia provide a window into early carbon cycling and microbe-mineral interactions. Here, we demonstrate the utility of correlative analytical approaches to study these rare fossils. Advances in electron microscopy and energy dispersive spectroscopy instrumentation reduce acquisition time, yet map critical elemental abundances at microscale resolution. When coupled with carbon isotopic measurements and nuclear magnetic resonance spectra, this approach enables us to distinguish primary depositional and secondary alteration features, improving interpretation of the fossil record.